

Similarly, in HF, a lower energy will be obtained if the valence electrons are biased toward the F centre. The relative energies of the atomic orbitals with n 4 for a hydrogen atom are plotted in Figure 2.5.8 note that the orbital energies depend on only the principal quantum number n. Hence, in the LiH molecule, we can already see that bonding electrons will be able to lower their energy by spending more time close to the H nucleus. Table 7.2 shows that the ionization potential of Li is 5.4 eV while that of H is 13.6 eV, and F has an even higher value of 18.6 eV. Īs examples of very polar bonds we will consider LiH and HF. So, we should expect that the greater the difference between the atomic ionization potentials of the atoms in a diatomic molecule, the more ionic the bond will be. The higher the energy required to remove an electron from an atom, the more strongly the electrons must be attracted to the atomic core.
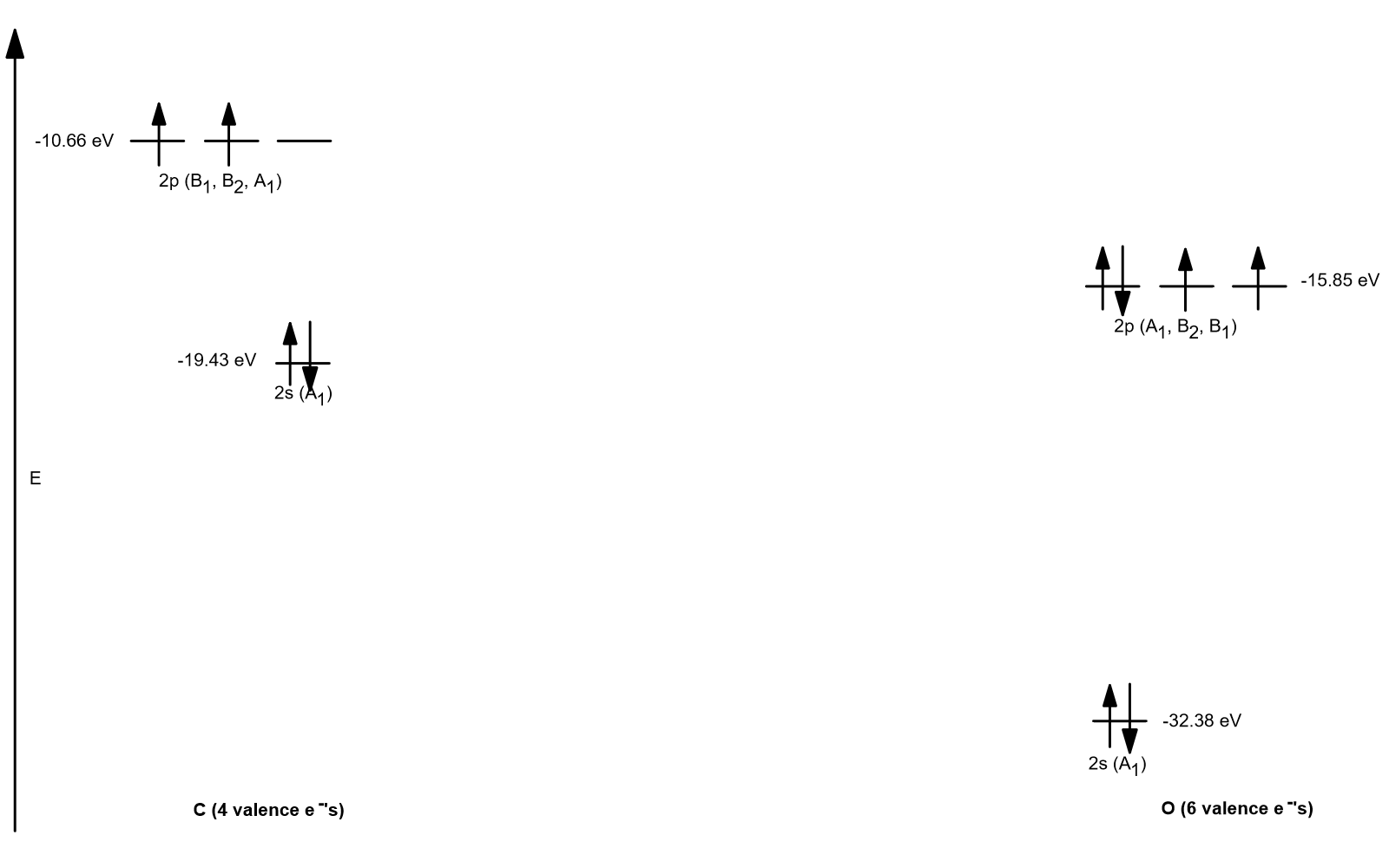
One measure of the relative attractive power is the ionization potential discussed in the previous section. In addition, the nuclear charges will be unequal, and so we may expect the electron density to be biased toward the more attractive of the two atomic cores. When atoms of different elements form a diatomic molecule, the relevant symmetry will be Coov because the symmetry elements which interchange the nuclei are no longer valid. composed of a single element), the sharing of electron density must be even between the two nuclei. In diatomic molecules with Dooh symmetry (i.e. In the previous example of H2 we saw that the covalent bond lowers the electronic energy by allowing the electrons to interact with more than one nucleus. 2. The Relative Energies of Atomic Orbitals from Electronegativityģ The Relative Energies of Atomic Orbitals from Electronegativity


 0 kommentar(er)
0 kommentar(er)
